In the fast-paced world of grocery/quick-service restaurant (QSR) retail, every second counts—especially when it comes to temperature control. Whether it’s keeping a frozen pizza solid in a sweltering delivery truck or making sure dairy stays fresh through a busy stocking shift, temperature management is a constant challenge. Behind the scenes, three key scientific concepts—specific heat capacity, thermal inertia and thermal conductivity—help explain how food responds to temperature changes. Combine those with the right Logile Thermal Intelligence(TM) (TI) technology, and you’ve got a powerful formula for better safety, lower shrink and smarter operations.
The Science Behind the Shelf
Let’s start with the basics.
Heat is a form of energy, specifically thermal energy, that is transferred between objects or systems due to a difference in temperature.
Temperature, on the other hand, is a measure of the average kinetic energy of the molecules within a substance, indicating how hot or cold something is. Important fact to remember: “Cold is not a substance or force, just a relative lack of heat (thermal energy).”
Specific heat capacity (SHC) is the amount of energy required to raise the temperature of a food product by one degree Celsius. Foods with high SHC—like milk, produce or raw meat—take longer to warm up. That can be a good thing during brief disruptions like a cooler door left open, but it also means they require more energy to chill or re-cool once exposed.
Specific Heat Capacity of Foods = A Sponge for Heat
Think of specific heat capacity like a sponge soaking up water—but instead of water, it’s heat.
- A big, thick sponge can soak up a lot of water before it drips.
- Similarly, a food with high specific heat (like milk or watermelon) can absorb a lot of heat before its temperature rises noticeably.
On the other hand:
- A small, thin sponge gets saturated quickly.
- Foods with low specific heat (like butter or bread) heat up quickly—they can’t “hold” as much energy without changing temperature.
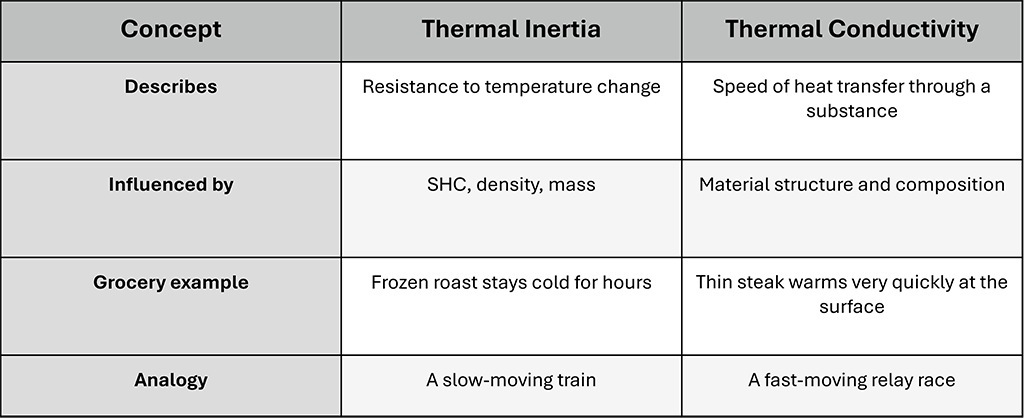
Thermal inertia is a concept that describes how resistant a product is to temperature change based not just on its composition, but also its mass. A 5-pound roast has far more thermal inertia than a tub of whipped topping. That roast will stay cold much longer if it’s left out—good news in a stocking rush. But once it does start to warm up, it will take time (and energy) to bring it back down safely.
Thermal conductivity is a measure of how quickly and easily heat moves through a material.
In simpler terms:
- It tells you how fast heat travels from the surface of a food item into its center (or vice versa).
- Foods with high thermal conductivity transfer heat fast.
- Foods with low thermal conductivity are more insulated and heat up (or cool down) slowly on the inside.
Thermal Inertia and Thermal Conductivity Side by Side
Here’s a clear and practical example that illustrates how the outside of a food product warms up faster than the inside, and why that creates a hidden food safety risk—especially important for grocery operations:
* Always measure the temperature of food products within the first 1/2” to 1”
Example: Ground Beef Chub on a Stocking Cart
You’ve got a 10-pound chub of ground beef sitting on a cart during a busy stocking shift. It’s been out of the cooler for 25 minutes.
- The center of the chub is still icy cold—maybe around 34°F (1°C) (High thermal inertia)
- But the outer inch has already climbed above 41°F (5°C)—the upper limit of the safe zone (Thermal conductivity)
- In some spots near the surface, it may even be reaching 50°F = Danger
Why? Because:
- The surface is exposed to warm air, so it absorbs heat quickly.
- Meat has moderate thermal conductivity, meaning the outer layers heat up long before the center catches up.
- High thermal inertia keeps the core cold, which can be misleading.
The Food Safety Danger:
- Bacteria grow rapidly on the warm outer surface—even while the inside still feels cold to the touch.
- If not rotated quickly or put back into refrigeration, that surface growth can multiply to unsafe levels, even though the product “feels fine.”
Refrigerated vs. Frozen: Not All Cold Is Equal
When it comes to cold storage, refrigerated and frozen foods behave very differently.
Refrigerated foods (kept around 0–4°C / 32–40°F) often have high SHC due to their water content. They’re slower to heat up, but once exposed to warmer conditions, they can quietly creep into the danger zone if not monitored.
Frozen foods are considered by many to be in a much more stable environment than refrigerated foods. This misconception could not be further from the truth. Frozen foods are thermally much more complex. Ice has a lower specific heat (50% LESS THAN WATER), meaning frozen products warm up faster after they begin to thaw. However, they also require a large amount of energy to initiate that thawing process—called the latent heat of fusion. This means that solid frozen foods often have a small buffer period before any real danger begins. Once they cross that line, though, the clock starts ticking fast.
Ice Cream: The Canary in the Freezer
Perhaps no product tells a better temperature story than ice cream. Its soft, scoopable texture depends on perfectly controlled ice crystals. Ice cream is especially sensitive to temperature swings due to its delicate balance of water, fat, sugar and air. When temperature fluctuates, the small ice crystals inside melt and refreeze into larger crystals. This process, called recrystallization, leads to a gritty, icy texture that indicates compromised quality and a clear signal that something’s wrong.
Ice cream, in many operations, serves as a first-alert indicator. If it’s showing signs of melt and refreeze, it’s likely other freezer items are also compromised. Operators who keep an eye on ice cream condition can often catch a failing unit before it turns into a full loss.
The Sensor Revolution: From Air Temp to Food Simulation
Traditional refrigeration systems rely heavily on air temperature sensors. But here’s the catch: air temperature changes rapidly—especially near open doors or defrost cycles—while the actual food temperature lags behind. That mismatch can cause false alarms or, worse, a failure to notice real product risk.
Enter food-simulating sensors like Logile TI. These devices are engineered to mimic how food itself reacts to temperature change, giving a far more accurate view of what’s really happening inside the case. They provide a truer read and reduce the chance of overcorrecting for harmless blips—or under-reacting to slow-rising risks.
From Theory to Action: What Grocery Teams Can Do
Here’s how grocery operators can turn science into savings:
- Use food-mimicking sensors instead of relying solely on air temperature.
- Train teams to understand thermal inertia—and why not all products warm up (or cool down) at the same rate.
- Monitor sensitive items like ice cream as early warning signs for freezer issues.
- Account for time + temperature together when evaluating risk. It’s not just about how warm—it’s about how long.
Final Scoop
Specific heat capacity, thermal conductivity and thermal inertia might sound like classroom science terms, but on the grocery floor, they’re the difference between safe, high-quality food and a costly recall. By combining this scientific insight with smarter monitoring tools like Logile TI thermal inertia sensors, operations teams can better protect their inventory, reduce waste, and ensure customers are getting the safest, freshest products possible.
So the next time you check the temperature of a case or spot icy buildup on a tub of vanilla, remember: it’s not just cold—it’s physics in action.
Recent Posts
How Technology Enables Retailers to Invest in Community-Centric In-Store Experiences
2026-01-05
Community programs need operational precision to succeed. See how Lowes Foods' Community Table thrives with AI-driven labor planning that creates capacity for connection.
Read More
NRF 2026 Guide: Top Trends Shaping the Future of Retail and What to Expect From Logile
2025-12-15
Logile celebrates 20 years at NRF 2026 with AI-powered workforce solutions. Visit Booth #4849 & #1503 to see how connected operations drive retail success.
Read More
UK Retail Budget 2025: What Retailers Need to Respond to Now
2025-12-02
The 2025 UK Budget is reshaping retail economics. Rising labour costs, higher rates, tighter margins—here's what retail leaders must prioritize now.
Read More

